Description de la soumission d'un avis
Institute of NeuroPhysiopathology

- Neural Plasticity and Degeneration
- Synaptic Degeneration and Gliosis
- Blood Brain Barrier and Neuroinflammation
- Oligodendroglial Cell Lineage in Aging and in Disease
- SInBioSE (Système nerveux, Inflammation et BIOmarqueurs de la Sclérose En plaques)
- Stem cells, Disease modeling and Neuroregeneration
- NeuroCyto: the neuronal cytoskeleton in health and disease
- GlioME: Gliomagenesis and MicroEnvironment
- Molecular Interactions and Pharmacology
- Angiogenesis and Tumor Microenvironment
- NOSE. Nasal Olfactory Stemness and Epigenesis
The Institute of NeuroPhysiopathology (INP), UMR7051 (CNRS-AMU) develops high-level fundamental, applied and translational research in neuroscience, leading to applications in the biomedical field, whether in industry or the clinic. The INP’s research strategy focuses on two main areas: neurodegenerative diseases and neuro-oncology, which are fueled by the study of cross-disciplinary mechanisms involved in neuronal ang glial cell plasticity, neuroinflammation and neurovascular interactions, cytoskeletal organization, the brain’s immune microenvironment, post-injury repair and learning and memory. These themes are underpinned by strong expertise developed by INP teams over the years in the fields of Alzheimer’s disease, glioblastoma, multiple sclerosis and cytoskeletal regulation at the molecular and cellular level.
The INP also includes 4 technology platforms (PFNT): The “Neuro-Cellular Imaging Support” (NCIS) imaging platform, accredited as a Nikon Center of Excellence, the “Timone Molecular Interactions Platform” (PINT), the “Stem Cell Center NeuroTimone” (SCeNT) platform, and the Preclinical and Translational Neuro-oncology Research platform (PETRA’Tech)
Together with the biotechnology company Vect-Horus (40 persons), the INP (120 persons) constitutes a Labcom (public-private partnership) dedicated to developing strategies against brain disease involving the crossing of the blood-brain barrier.
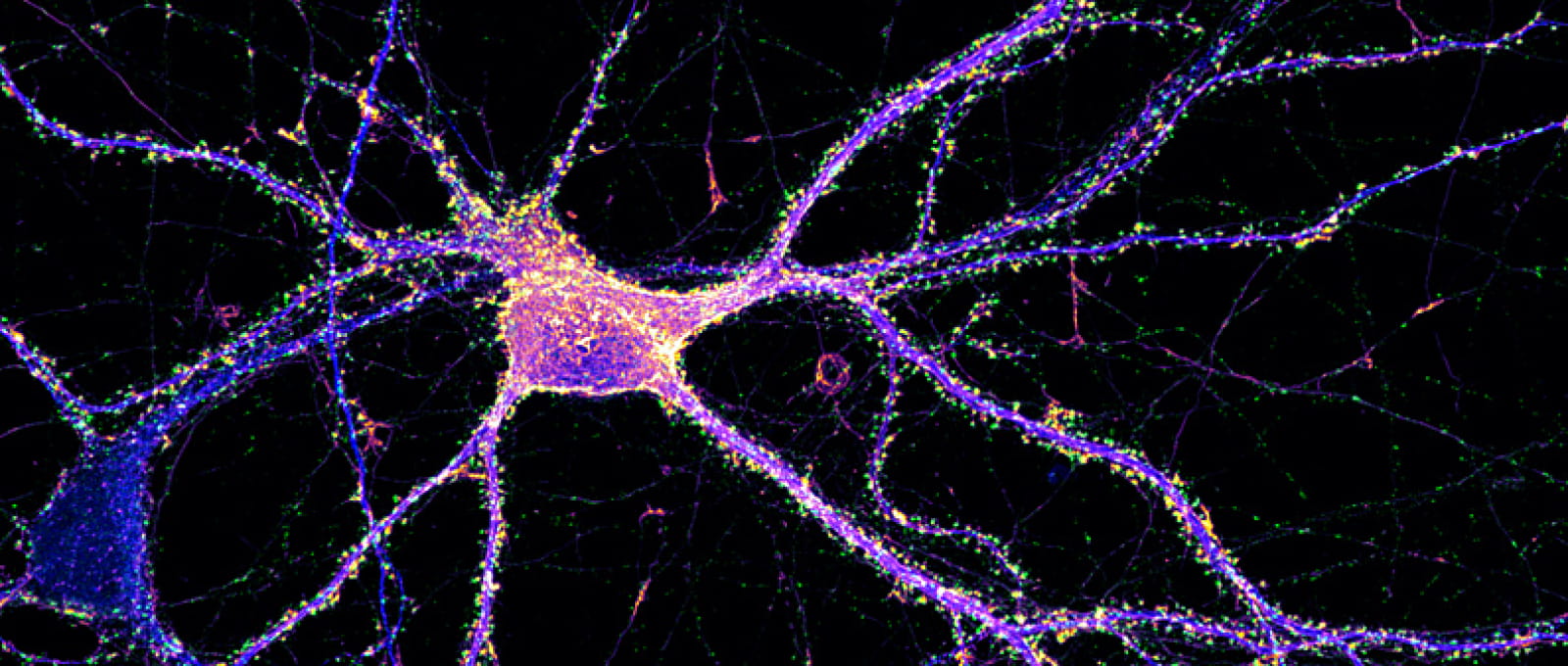
Pictures from the INP laboratory



INP platforms
Neural Plasticity and Degeneration
DescriptionTo find out more, click on the following link : here
Santiago Rivera
Synaptic Degeneration and Gliosis
DescriptionTo find out more, click on the following link : here
Maud Gratuze
Blood Brain Barrier and Neuroinflammation
DescriptionTo find out more, click on the following link : here
Michel Khrestchatisky
Oligodendroglial Cell Lineage in Aging and in Disease
DescriptionTo find out more, click on the following link : here
Sarah Moyon
SInBioSE (Système nerveux, Inflammation et BIOmarqueurs de la Sclérose En plaques)
DescriptionTo find out more, click on the following link : here
Sophie Desplat-Jégo
Stem cells, Disease modeling and Neuroregeneration
DescriptionTo find out more, click on the following link : here
Emmanuel Nivet
NeuroCyto: the neuronal cytoskeleton in health and disease
DescriptionTo find out more, click on the following link : here
Christophe Leterrier
GlioME: Gliomagenesis and MicroEnvironment
DescriptionTo find out more, click on the following link : here
Aurélie Tchoghandjian
Emeline Tabouret
Molecular Interactions and Pharmacology
DescriptionTo find out more, click on the following link : here
Hervé Kovacic
François Devred
Angiogenesis and Tumor Microenvironment
DescriptionTo find out more, click on the following link : here
L'Houcine Ouafik
NOSE. Nasal Olfactory Stemness and Epigenesis
DescriptionTo find out more, click on the following link : here
Gaëlle Guiraudie-Capraz


